The research in NECO lab mostly generally concerns neuromodulation of cognitive processes, with a primary focus on attention and executive functions. These mental processes are critical for learning, acquiring and assimilating knowledge through experience, and applying this knowledge to act sensibly in changing environments. Cognitive abilities decline in patients suffering from age-related neurodegenerative disorders and major psychiatric disorders, impacting individuals’ ability to perform activities of daily living, work productively and function socially. Our research program incorporates both bottom-up (basic science) and top-down (translational science) perspectives to achieve a better understanding of the neural substrates critical to maintain cognitive health and of the fundamental mechanisms underlying cognitive dysfunction associated with brain pathologies. This research is important because identifying the causal mechanisms along with potential biomarkers may allow us to design interventions to treat cognitive disorders. We use an interdisciplinary approach that use diverse neuroscientific tools including vector-based RNAi, chemogenetics, transcriptomics, protein biochemistry, biosensor-based electrochemical detection, and cell imaging, along with the assessment of complex behaviors.
Attentional capacities and aging cholinergic system
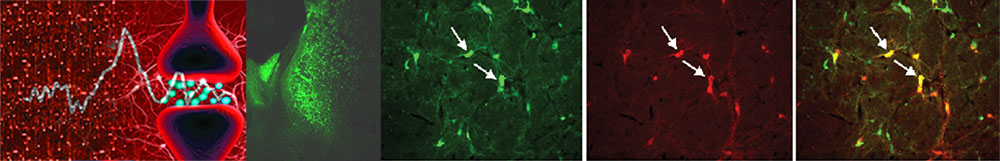
Attention describes a set of cognitive processes that support the ability to selectively concentrate on one aspect of the environment while ignoring others. These selective processes facilitate our ability to encode, organize, store, revise, and retrieve information. Much of our previous research focused on determining the contribution of forebrain cholinergic system in attention. We discovered that cortical cholinergic signaling operates in multiple modes (phasic and tonic) to encode information pertaining to cues guiding behavior, to optimize signal detection and to maintain sensory arousal. At the molecular level, we found that the trafficking of the high-affinity choline transporter (CHT) proteins is critical to maintain phasic cholinergic transmission under attention-demanding conditions. Attentional impairments are visible during the earlier stages of Alzheimer’s disease (AD), and are proposed to contribute to progressive impairments in episodic memory and executive functions. As aging is a well-recognized risk factor for AD, understanding how cholinergic modulation of attention is regulated in normal and pathological aging is important. We examined the contributions of cholinotrophic protein nerve growth factor (NGF) signaling in cholinergic modulation of attention in aging. Our research suggests that a balance between prosurvival NGF-trkA and death-inducing proNGF-p75 signaling pathways is critical to maintain phasic cholinergic signaling in normal aging, and that suppression of neuroprotective signaling pathways disrupts attentional capacities by compromising the ability of aging cholinergic neurons to sustain presynaptic activity. In addition, our studies also highlighted that detrimental interactions between soluble Aβ oligomers and CHT trafficking mechanisms disrupts phasic cholinergic transients and transporter-driven clearance mechanisms which may underlie attentional impairments in early AD. Collectively, our findings suggest cholinergic signaling and attentional capacities are differentially regulated in normal and pathological aging. Moreover, this research has provided a framework to exploit new biological targets to develop therapeutic interventions that preserve synaptic cholinergic signaling and halt cognitive deterioration in AD.
Neural substrates of cognitive resilience and vulnerability in aging
Although individual differences in cognitive aging have been observed, it is not clear why certain individuals show enhanced vulnerability to cognitive decline and eventually develop age-related dementias and AD, while others exhibit resilience to age-related cognitive pathologies. Using the PASA framework, we investigated whether differences in neuroadaptive capacity in cortical networks underlie age-related cognitive variability. Our results show intra- and inter-hemispheric cortical shifts in aging are not compensatory, suggesting reduced efficiency and deleterious changes in prefrontal cortex-mediated top-down control may underlie cognitive impairments in vulnerable subjects. We also found that excitation-inhibition balance in the local circuits of reorganized cortical networks is primarily regulated by cholinergic mechanisms which may provide a reserve mechanism to preserve the efficiency and performance. Using high-throughput RNA-seq approaches, we recently discovered that transcriptomes of innate immune signaling and extracellular exosomes may determine network efficiency and cognitive variability in aging. Ongoing research is examining how dynamic interactions between genes and environment (e.g. cognitive enrichment, stressful experiences) shape the vulnerability and resilience to age-related decline in cognitive and mental capacities. Specifically, we are investigating transcriptional and post-translational mechanisms that underlie long-term cholinergic plasticity and cholinergic modulation of neuroimmune pathways in aging.
Predictive biomarkers of age-related cognitive decline
With increasing aging population, the incidence of age-related dementia and AD is becoming more prominent. Currently, there are no effective treatments that could prevent these pathologies, halt their progression or delay their onset, and efforts to develop biomarkers may facilitate improved diagnosis and design an early mechanism-based therapeutic intervention. Our ongoing behavioral, neurochemical, and qEEG studies in preclinical models are aimed to identify potential cognitive endophenotypes and neurobehavioral biomarkers that may predict the risk of developing cognitive impairments in pathological aging. We are also utilizing predictive modeling approaches on human datasets to identify key predictors of cognitive decline in older adults.
Frontrostriatal system adaptations and cognitive control
Adaptive cognitive control is necessary to act flexibly in changing environments and to maintain cognitive operations in accordance with ongoing task demands. These executive processes are critical to regulate motivation to achieve goals. The integrity of frontostriatal circuits is critical for cognitive flexibility and these circuits are compromised in neuropsychiatric disorders. Our previous research illustrated that glutamate signaling in the prefrontal cortex and in the downstream striatal targets of prefrontal neurons is the prime orchestrator of attentional and executive control, and the dynamics of these neurochemical circuits is altered with chronic use of stimulant drugs. We also identified brain-derived neurotrophic factor (BDNF) as a key molecular substrate that regulate corticostriatal glutamate signaling, optimize glutamate-dopamine interactions, and maintain cognitive flexibility. Using preclinical models of nicotine addiction, our recent research show nicotine abstinence impairs distinct components of cognitive set-shifting, and these deficits are linked to BDNF-trkB signaling and downstream perturbations in synapsin phosphorylation. These findings provide fundamental insights into the neurocognitive mechanisms of nicotine addiction. Because neurotrophin signaling is also implicated in major neuropsychiatric conditions including schizophrenia, and the drug use is common in these disorders, our research also open avenues to develop psychotherapeutic interventions for comorbid conditions.
Collaborators
Debra Bangasser, Ph.D. (Temple University)
Lisa Briand, Ph.D. (Temple University)
Thomas J. Gould, Ph.D. (Penn State University)
Scott M. Rawls, Ph.D. (Temple University School of Medicine)
Martin Sarter, Ph.D. (University of Michigan)
David L. Turner, Ph.D. (University of Michigan)
Ellen M. Unterwald, Ph.D. (Temple University School of Medicine)
Mathieu Wimmer, Ph.D. (Temple University)
Angela J. Yu, Ph.D. (University of California, San Diego)