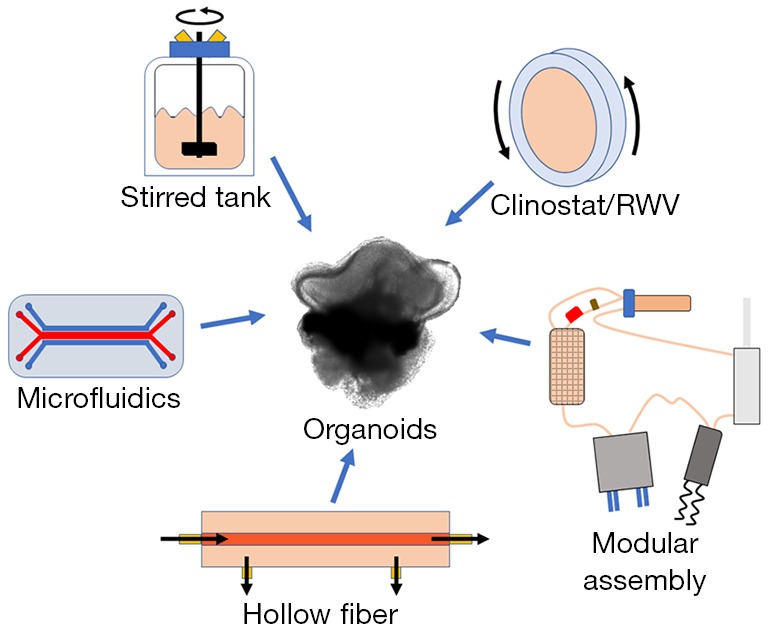
Bioreactor Fabrication for Organoid Culture
Our lab designs and uses custom bioreactors for organoid development and stem cell differentiation. Current research involves creating systems using modified HARVs (High Aspect Ratio Vessels) as well as novel devices for use in a Random Positioning Machine. Both are devices meant to model microgravity.
3D Bioprinting
Bioprinting is an amazing technology that allows researchers to create complex biomimetic structures.

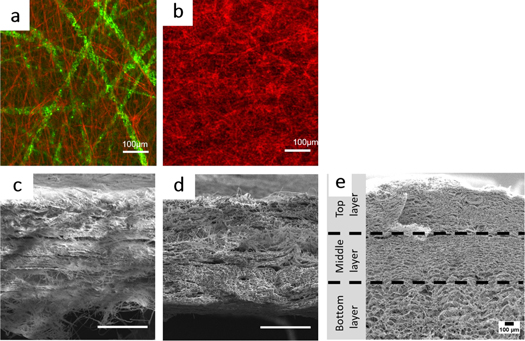
Electrospinning Organic Scaffolds
This project involves using electrospinning/electroblowing techniques to create fibrous scaffolds out of organic molecules, primarily soy. These can be used for skin would healing as well as bone regeneration.
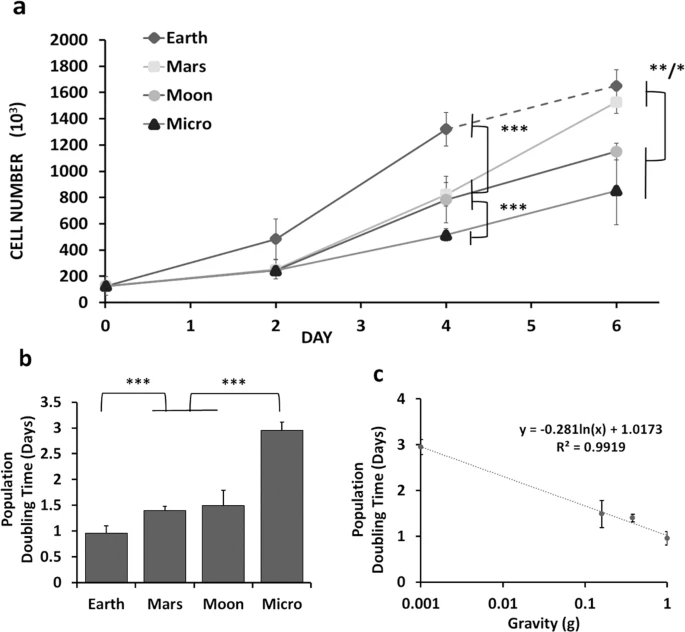
Effects of altered gravity on osteogenic cells
We study the effects that low gravity have on osteogenic (bone) cells in the hopes of rescuing the effect that low gravity has on an astronaut’s bones.
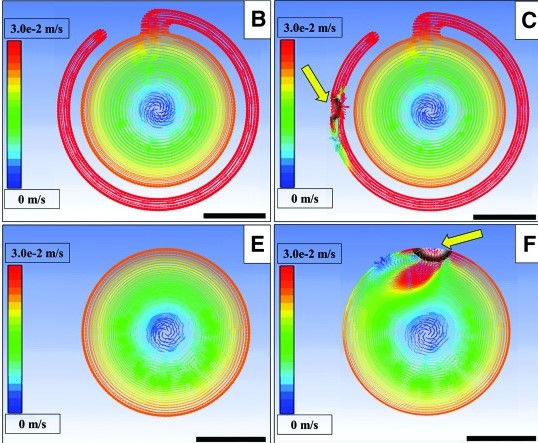
Computational Modeling
We perform computation modeling for many purposes, including:
– Stem cell growth and differentiation
– 3D cell aggregation
– Bioreactor fluid dynamics
– Electric field simulation for bioreactors and electrospinning
Recent Publications
- Lazarovici, P., Marcinkiewicz, C., & Lelkes, P.I. (2020). Cell-based adhesion assays for isolation of snake venom’s integrin antagonists., 2068, pp. 205-223. doi: 10.1007/978-1-4939-9845-6_11
- Goulart, E., Caires-Junior, L.C.D.e., Telles-Silva, K.A., Araujo, B., Rocco, S.A., Sforca, M., Sousa, I.L.D.e., Kobayashi, G.S., Musso, C.M., Assoni, A.F., Oliveira, D., Caldini, E., Raia, S., Lelkes, P.I., & Zatz, M. (2020). 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication, 12(1). doi: 10.1088/1758-5090/ab4a30
- Du, Y., Montoya, C., Orrego, S., Wei, X., Ling, J., Lelkes, P.I., & Yang, M. (2019). Topographic cues of a novel bilayered scaffold modulate dental pulp stem cells differentiation by regulating YAP signalling through cytoskeleton adjustments. Cell Proliferation, 52(6). doi: 10.1111/cpr.12676
- Goulart, E., Caires-Junior, L.C.D.e., Telles-Silva, K.A., Araujo, B., Kobayashi, G.S., Musso, C.M., Assoni, A.F., Oliveira, D., Caldini, E., Gerstenhaber, J.A., Raia, S., Lelkes, P.I., & Zatz, M. (2019). Adult and iPS-derived non-parenchymal cells regulate liver organoid development through differential modulation of Wnt and TGF-ß. Stem Cell Research and Therapy, 10(1). doi: 10.1186/s13287-019-1367-x
- Malik, R., Luong, T., Cao, X., Han, B., Shah, N., Franco-Barraza, J., Han, L., Shenoy, V.B., Lelkes, P.I., & Cukierman, E. (2019). Rigidity controls human desmoplastic matrix anisotropy to enable pancreatic cancer cell spread via extracellular signal-regulated kinase 2. Matrix Biology, 81, pp. 50-69. doi: 10.1016/j.matbio.2018.11.001
- Phelan, M.A., Gianforcaro, A.L., Gerstenhaber, J.A., & Lelkes, P.I. (2019). An Air Bubble-Isolating Rotating Wall Vessel Bioreactor for Improved Spheroid/Organoid Formation. Tissue Engineering – Part C: Methods, 25(8), pp. 479-488. doi: 10.1089/ten.tec.2019.0088
- Lazarovici, P., Marcinkiewicz, C., & Lelkes, P.I. (2019). From snake venom’s disintegrins and C-type lectins to anti-platelet drugs. Toxins, 11(5). doi: 10.3390/toxins11050303
- Novosel, E., Borchers, K., Kluger, P.J., Mantalaris, A., Matheis, G., Pistolesi, M., Schneider, J., Wenz, A., & Lelkes, P.I. (2019). New approaches to respiratory assist: Bioengineering an ambulatory, miniaturized bioartificial lung. ASAIO Journal, 65(5), pp. 422-429. doi: 10.1097/MAT.0000000000000841
- Barone, F.C., Marcinkiewicz, C., Li, J., Feng, Y., Sternberg, M., Lelkes, P.I., Rosenbaum-Halevi, D., Gerstenhaber, J.A., & Feuerstein, G.Z. (2019). Long-term biocompatibility of fluorescent diamonds-(NV)-Z~800 nm in rats: Survival, morbidity, histopathology, particle distribution and excretion studies (part IV). International Journal of Nanomedicine, 14, pp. 1163-1175. doi: 10.2147/IJN.S189048
- Gerstenhaber, J.A., Marcinkiewicz, C., Barone, F.C., Sternberg, M., D’Andrea, M.R., Lelkes, P.I., & Feuerstein, G.Z. (2019). Biocompatibility studies of fluorescent diamond particles-(Nv)~800nm (part v): In vitro kinetics and in vivo localization in rat liver following long-term exposure. International Journal of Nanomedicine, 14, pp. 6451-6464. doi: 10.2147/IJN.S209663
- Phelan, M.A., Lelkes, P.I., & Swaroop, A. (2018). Mini and customized low-cost bioreactors for optimized high-throughput generation of tissue organoids. Stem Cell Investigation, 5(October). doi: 10.21037/sci.2018.09.06
- Timnak, A., Gerstenhaber, J.A., Dong, K., Har-El, Y.E., & Lelkes, P.I. (2018). Gradient porous fibrous scaffolds: A novel approach to improving cell penetration in electrospun scaffolds. Biomedical Materials (Bristol), 13(6). doi: 10.1088/1748-605X/aadbbe
- Senel-Ayaz, H.G., Har-El, Y.E., Ayaz, H., & Lelkes, P.I. (2018). Textile technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications (pp. 175-201). doi: 10.1016/B978-0-08-100979-6.00008-2
- Lelkes, P.I. (2018). Methodological aspects dealing with stability measurements of liposomes in vitro using the carboxyfluoresceinassay. In Liposome Technology Volume III: Targeted Drug Delivery and Biological Interaction (pp. 225-246). doi: 10.1201/9781351074117
- Barone, F.C., Gerstenhaber, J.A., Marcinkiewicz, C., Li, J., Lelkes, P.I., Sternberg, M., & Feuerstein, G.Z. (2018). Imaging Intra-Carotid Thrombosis Using Near InfraRed Fluorescent-NanoDiamond Particles Bio-engineered With the Disintegrin Bitistatin. STROKE, 49. Retrieved from http://gateway.webofknowledge.com/
- Barone, F.C., Marcinkiewicz, C., Li, J., Sternberg, M., Lelkes, P.I., Dikin, D.A., Bergold, P.J., Gerstenhaber, J.A., & Feuerstein, G. (2018). Pilot study on biocompatibility of fluorescent nanodiamond-(NV)-Z~800 particles in rats: Safety, pharmacokinetics, and bio-distribution (Part III). International Journal of Nanomedicine, 13, pp. 5449-5468. doi: 10.2147/IJN.S171117
- Lelkes, P.I. (2018). Methodological aspects dealing with stability measurements of liposomes in vitro using the carboxyfluoresceinassay. In Liposome Technology: Volume III: Targeted Drug Delivery and Biological Interaction (pp. 225-246). doi: 10.1201/9781351074124
- Lazarovici, P., Lahiani, A., Gincberg, G., Haham, D., Fluksman, A., Benny, O., Marcinkiewicz, C., & Lelkes, P.I. (2018). Nerve growth factor-induced angiogenesis: 1. Endothelial cell tube formation assay. In Methods in Molecular Biology, 1727 (pp. 239-250). doi: 10.1007/978-1-4939-7571-6_18
- Lazarovici, P., Lahiani, A., Gincberg, G., Haham, D., Marcinkiewicz, C., & Lelkes, P.I. (2018). Nerve growth factor-induced angiogenesis: 2. The quail chorioallantoic membrane assay. In Methods in Molecular Biology, 1727 (pp. 251-259). doi: 10.1007/978-1-4939-7571-6_19
- Timnak, A., Har-el, Y., & Lelkes, P.I. (2017). Macrophage Infiltration into Macro-porous Electrospun Scaffolds Initiates a Reparative Inflammatory Response. TISSUE ENGINEERING PART A, 23, pp. S41-S41. Retrieved from http://gateway.webofknowledge.com/
- Gerstenhaber, J.A., Barone, F.C., Marcinkiewicz, C., Li, J., Shiloh, A.O., Sternberg, M., Lelkes, P.I., & Feuerstein, G. (2017). Vascular thrombus imaging in vivo via near-infrared fluorescent nanodiamond particles bioengineered with the disintegrin bitistatin (Part II). International Journal of Nanomedicine, 12, pp. 8471-8482. doi: 10.2147/IJN.S146946
- Marcinkiewicz, C., Gerstenhaber, J.A., Sternberg, M., Lelkes, P.I., & Feuerstein, G. (2017). Bitistatin-functionalized fluorescent nanodiamond particles specifically bind to purified human platelet integrin receptor aiibß3 and activated platelets. International Journal of Nanomedicine, 12, pp. 3711-3720. doi: 10.2147/IJN.S134128
- Kischkel, S., Bergt, S., Brock, B., Grönheim, J.V., Herbst, A., Epping, M.J., Matheis, G., Novosel, E., Schneider, J., Warnke, P., Podbielski, A., Roesner, J.P., Lelkes, P.I., & Vollmar, B. (2017). In Vivo Testing of Extracorporeal Membrane Ventilators: ILA-Activve Versus Prototype I-Lung. ASAIO Journal, 63(2), pp. 185-192. doi: 10.1097/MAT.0000000000000465
- Devlin, S.M., Gangolli, R.A., Spangenberg, N., Batish, R., Hagaman, D., Ji, H., & Lelkes, P.I. (2016). Improving Degradable Biomaterials for Orthopedic Fixation Devices. TISSUE ENGINEERING PART A, 22, pp. S147-S147. Retrieved from http://gateway.webofknowledge.com/
- Gangolli, R.A., Devlin, S.M., Ailavajhala, R., Hanifi, A., Pleshko, N., Lelkes, P.I., & Yang, M. (2016). Biomimetic Scaffold to Regenerate the Pulp Dentin Complex. TISSUE ENGINEERING PART A, 22, pp. S72-S72. Retrieved from http://gateway.webofknowledge.com/
- Wass, B.M., Lelkes, P.I., Stabler, C.T., & Garcia, R. (2016). Model of Microvascular Pulmonary Inflammation Modulated by Extracellular Matrix Mechanical Properties. TISSUE ENGINEERING PART A, 22, pp. S66-S66. Retrieved from http://gateway.webofknowledge.com/
- Palukuru, U.P., Hanifi, A., McGoverin, C.M., Devlin, S., Lelkes, P.I., & Pleshko, N. (2016). Near infrared spectroscopic imaging assessment of cartilage composition: Validation with mid infrared imaging spectroscopy. Analytica Chimica Acta, 926, pp. 79-87. doi: 10.1016/j.aca.2016.04.031
- Stabler, C.T., Caires, L.C., Mondrinos, M.J., Marcinkiewicz, C., Lazarovici, P., Wolfson, M.R., & Lelkes, P.I. (2016). Enhanced Re-Endothelialization of Decellularized Rat Lungs. Tissue Engineering – Part C: Methods, 22(5), pp. 439-450. doi: 10.1089/ten.tec.2016.0012
- Hindin, D., Baharlou, S.M., Gerstenhaber, J., Lo, T.Y., Har-El, Y., Bradley, J.P., & Lelkes, P.I. (2015). Electrospun soy protein scaffolds enhance skin regeneration in a rat wound model. JOURNAL of the AMERICAN COLLEGE of SURGEONS, 221(4), pp. E117-E118. doi: 10.1016/j.jamcollsurg.2015.08.213
- Gerstenhaber, J.A., Har-el, Y., & Lelkes, P.I. (2015). Electrospinning of Personalized Scaffolds for Wound Healing by Robotic Electrospinner. Biomedical Engineering Society Meeting.
- Har-el, Y., Gerstanhaber, J.A., Baharlou, S.M., Lo, T.Y., Hindin, D., Brodsky, R., Huneke, R.B., & Lelkes, P.I. (2015). Bioactive Alimentary Protein-Based Scaffolds (APS) Enhance Wound Healing. PA BIO Life Sciences Future Event.
- Gangolli, R.A., Devlin, S.M., Gerstenhaber, J.A., Lelkes, P.I., & Yang, M. (2015). Biomimetic Scaffold for Guided Interfacial Tissue Regeneration in Regenerative Dentistry. TISSUE ENGINEERING PART A, 21, pp. S348-S348. Retrieved from http://gateway.webofknowledge.com/
- Karamil, S., Lazarovici, P., Fink, C., & Lelkes, P.I. (2015). Soft Tissue Stiffness Range Influences Early Commitment of Mouse Embryonic Stem Cells Towards Endodermal Lineage. TISSUE ENGINEERING PART A, 21, pp. S281-S281. Retrieved from http://gateway.webofknowledge.com/
- Guimaraes, E.G., Stabler, C.T., Tabler, M.N., Junior, L.C., Lecht, S., & Lelkes, P.I. (2015). Differentiation of Mouse Embryonic Stem Cells in Simulated Microgravity. TISSUE ENGINEERING PART A, 21, pp. S268-S268. Retrieved from http://gateway.webofknowledge.com/
- Ayaz, G.S., Ayaz, H., Perets, A., Govindaraj, M., Brookstein, D., & Lelkes, P. (2015). Generation and Functional in vitro Evaluation of Textile-templated Anisotropic Elastomeric Scaffolds for Cardiac Tissue Engineering. TISSUE ENGINEERING PART A, 21, pp. S383-S384. Retrieved from http://gateway.webofknowledge.com/
- Novosel, E.C., Matheis, G., Schneider, J., Wenz, A., Kluger, P., Borchers, K., & Lelkes, P. (2015). Development of a Cell-seeded, Bioartificial Lung Assist Device. TISSUE ENGINEERING PART A, 21, pp. S146-S146. Retrieved from http://gateway.webofknowledge.com/
- Stabler, C.T., Junior, L.C., Marcinkiewicz, C., & Lelkes, P. (2015). Integrin Specific Re-endothelialization within Decellularized Lungs. TISSUE ENGINEERING PART A, 21, pp. S85-S86. Retrieved from http://gateway.webofknowledge.com/
- Stabler, C.T., Lecht, S., Lazarovici, P., & Lelkes, P.I. (2015). Mesenchymal stem cells for therapeutic applications in pulmonary medicine. British Medical Bulletin, 115(1), pp. 45-56. doi: 10.1093/bmb/ldv026
- Malik, R., Luong, T., Karamil, S., Lelkes, P., & Cukierman, E. (2015). Desmoplastic stroma affects growth and invasion of progressively mutated human pancreatic cancer cells in vitro. CANCER RESEARCH, 75. doi: 10.1158/1538-7445.AM2015-5089
- Har-el, Y., Gerstenhaber, J.A., Baharlou, S.M., Lo, T.Y., Hindin, D., Brodsky, R., Huneke, R.B., & Lelkes, P.I. (2015). Bioactive Alimentary Protein-Based Scaffolds (APS) Enhance Wound Healing. BIO International Convention.
- Ventresca, E.M., Lecht, S., Jakubowski, P., Chiaverelli, R.A., Weaver, M., Valle, L.D., Ettinger, K., Gincberg, G., Priel, A., Braiman, A., Lazarovici, P., Lelkes, P.I., & Marcinkiewicz, C. (2015). Association of p75NTR and a9ß1 integrin modulates NGF-dependent cellular responses. Cellular Signalling, 27(6), pp. 1225-1236. doi: 10.1016/j.cellsig.2015.02.029
- Weiss, D.J., Chambers, D., Giangreco, A., Keating, A., Kotton, D., Lelkes, P.I., Wagner, D.E., & Prockop, D.J. (2015). An official American Thoracic Society workshop report: Stem cells and cell therapies in lung biology and diseases. Annals of the American Thoracic Society, 12(4), pp. S79-S97. doi: 10.1513/AnnalsATS.201502-086ST
- Frohbergh, M.E. & Lelkes, P.I. (2015). Biomimetic Scaffolds for Craniofacial Bone Tissue Engineering: Understanding the Role of the Periosteum in Regeneration. In Mechanical Engineering Series (pp. 147-165). Springer International Publishing. doi: 10.1007/978-3-319-13266-2_9
- Stabler, C.T., Lecht, S., Mondrinos, M.J., Goulart, E., Lazarovici, P., & Lelkes, P.I. (2015). Revascularization of decellularized lung scaffolds: Principles and progress. American Journal of Physiology – Lung Cellular and Molecular Physiology, 309(11), pp. L1273-L1285. doi: 10.1152/ajplung.00237.2015
- Malik, R., Lelkes, P.I., & Cukierman, E. (2015). Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends in Biotechnology, 33(4), pp. 230-236. doi: 10.1016/j.tibtech.2015.01.004
- Lecht, S., Chiaverelli, R.A., Gerstenhaber, J., Calvete, J.J., Lazarovici, P., Casewell, N.R., Harrison, R., Lelkes, P.I., & Marcinkiewicz, C. (2015). Anti-angiogenic activities of snake venom CRISP isolated from Echis carinatus sochureki. Biochimica Et Biophysica Acta – General Subjects, 1850(6), pp. 1169-1179. doi: 10.1016/j.bbagen.2015.02.002
- Frohbergh, M.E., Katsman, A., Mondrinos, M.J., Stabler, C.T., Hankenson, K.D., Oristaglio, J.T., & Lelkes, P.I. (2015). Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds. Tissue Engineering – Part A, 21(5-6), pp. 970-981. doi: 10.1089/ten.tea.2013.0789
- Pimton, P., Lecht, S., Stabler, C.T., Johannes, G., Schulman, E.S., & Lelkes, P.I. (2015). Hypoxia enhances differentiation of mouse embryonic stem cells into definitive endoderm and distal lung cells. Stem Cells and Development, 24(5), pp. 663-676. doi: 10.1089/scd.2014.0343