Integrated Laboratory for Cellular Tissue
Engineering and Regenerative Medicine
Housed in the Temple University College of Engineering, Department of Bioengineering since 2012. Headed by Dr. Peter Lelkes, Bioengineering Department Chair





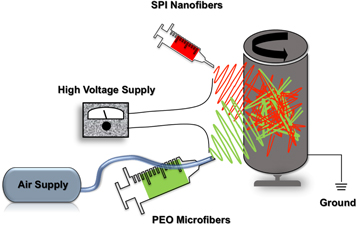
Current Featured Articles
Bioreactor Technologies for Enhanced Organoid Culture
by Joseph P. Licata, Kyle H. Schwab, Yah-el Har-el, Jonathan A. Gerstenhaber, and Peter I. Lelkes
Int. J. Mol. Sci. 2023, 24(14), 11427; https://doi.org/10.3390/ijms241411427
Abstract
An organoid is a 3D organization of cells that can recapitulate some of the structure and function of native tissue. Recent work has seen organoids gain prominence as a valuable model for studying tissue development, drug discovery, and potential clinical applications. The requirements for the successful culture of organoids in vitro differ significantly from those of traditional monolayer cell cultures. The generation and maturation of high-fidelity organoids entails developing and optimizing environmental conditions to provide the optimal cues for growth and 3D maturation, such as oxygenation, mechanical and fluidic activation, nutrition gradients, etc. To this end, we discuss the four main categories of bioreactors used for organoid culture: stirred bioreactors (SBR), microfluidic bioreactors (MFB), rotating wall vessels (RWV), and electrically stimulating (ES) bioreactors. We aim to lay out the state-of-the-art of both commercial and in-house developed bioreactor systems, their benefits to the culture of organoids derived from various cells and tissues, and the limitations of bioreactor technology, including sterilization, accessibility, and suitability and ease of use for long-term culture. Finally, we discuss future directions for improvements to existing bioreactor technology and how they may be used to enhance organoid culture for specific applications.
Featured Figure:
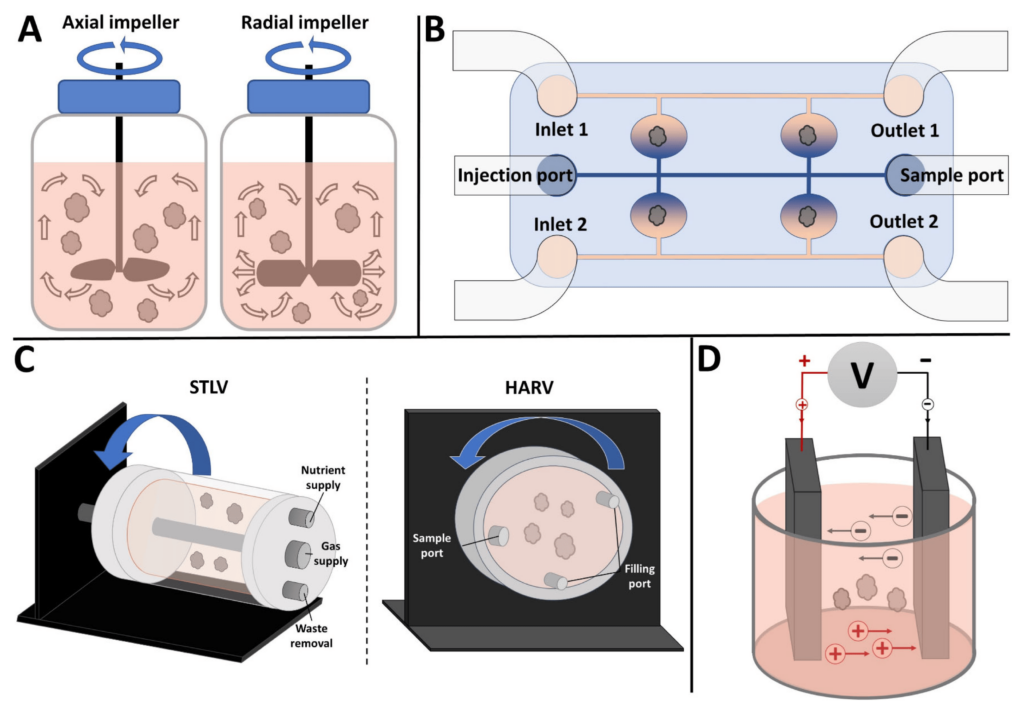
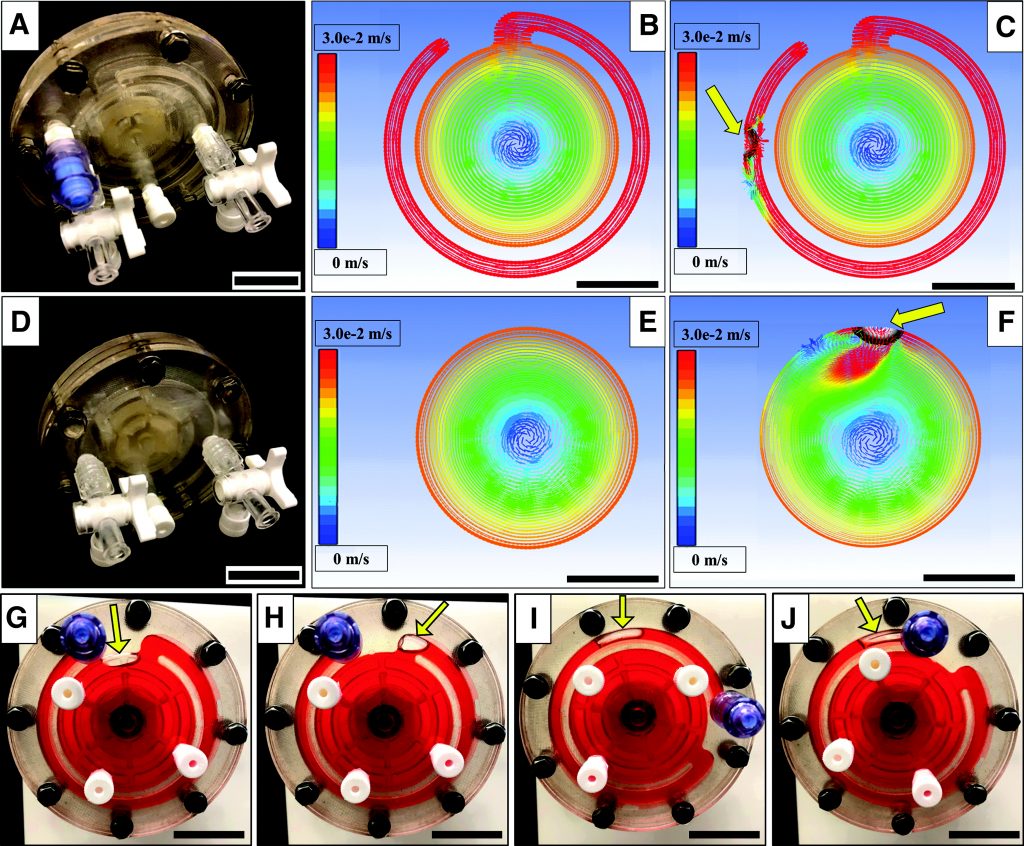
Figure 1. Commonly used bioreactors for organoid culture. (A) Stirred bioreactor with axial impeller (left) and radial impeller (right). Arrows show direction of fluid movement. (B) Microfluidic bioreactor with separate channels for perfusing different media. (C) Rotating wall vessel (RWV) bioreactors: (left panel) Slow Turning Lateral Vessel (STLV) and (right panel) High Aspect Ratio Vessel (HARV). Arrows show direction of rotation. (D) Electrical stimulation bioreactor showing two parallel plate electrodes. For details, see text.
Impairment of 7F2 osteoblast function by simulated partial gravity in a Random Positioning Machine
Justin Braveboy-Wagner, Peter I. Lelkes
Published: June 2022. npj Microgravity volume 8, Article number: 20 (2022) https://doi.org/10.1038/s41526-022-00202-x
Abstract
The multifaceted adverse effects of reduced gravity pose a significant challenge to human spaceflight. Previous studies have shown that bone formation by osteoblasts decreases under microgravity conditions, both real and simulated. However, the effects of partial gravity on osteoblasts’ function are less well understood. Utilizing the software-driven newer version of the Random Positioning Machine (RPMSW), we simulated levels of partial gravity relevant to future manned space missions: Mars (0.38 G), Moon (0.16 G), and microgravity (Micro, ~10−3 G). Short-term (6 days) culture yielded a dose-dependent reduction in proliferation and the enzymatic activity of alkaline phosphatase (ALP), while long-term studies (21 days) showed a distinct dose-dependent inhibition of mineralization. By contrast, expression levels of key osteogenic genes (Alkaline phosphatase, Runt-related Transcription Factor 2, Sparc/osteonectin) exhibited a threshold behavior: gene expression was significantly inhibited when the cells were exposed to Mars-simulating partial gravity, and this was not reduced further when the cells were cultured under simulated Moon or microgravity conditions. Our data suggest that impairment of cell function with decreasing simulated gravity levels is graded and that the threshold profile observed for reduced gene expression is distinct from the dose dependence observed for cell proliferation, ALP activity, and mineral deposition. Our study is of relevance, given the dearth of research into the effects of Lunar and Martian gravity for forthcoming space exploration.
Featured Figure:
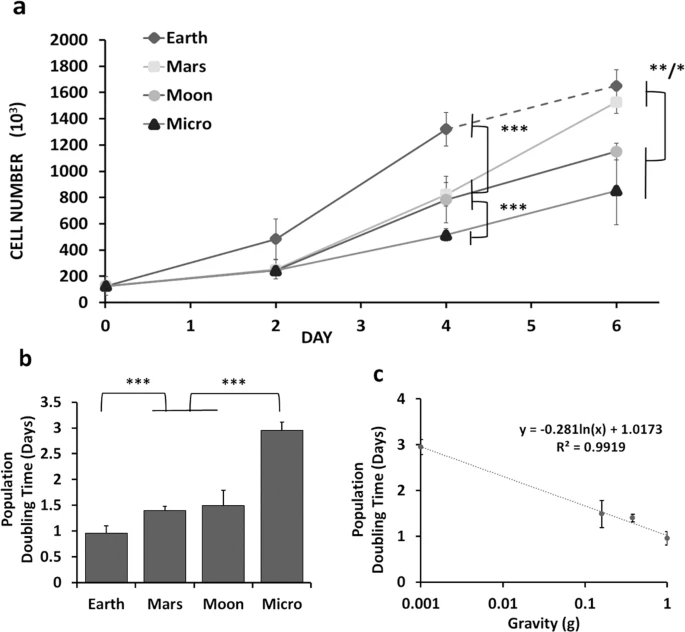
a Increase in cell numbers on days 2 and 4 of culture in 1 G (Earth) and under the various altered simulated partial gravity conditions (Mars, Moon, Micro) (N = 3). b Specific partial population doubling times between days 2 and 4 and taking into account the mean absolute error (MAE). The average population doubling times were calculated as 0.96 ± 0.14 days for Earth (1 G), 1.4 ± 0.08 days for Mars, 1.5 ± 0.30 days for Moon, and 2.95 ± 0.17 days for simulated microgravity (Micro). c Semilogarithmic plot of the specific population doubling times vs. simulated partial gravity (R2 = 0.9919) for that 48-h window of time. Data are presented as means ± standard deviation. Asterisk (*) shows p < 0.05, (**) shows p < 0.01, (***) p < 0.001 as determined by Tukey’s post hoc analysis (panels (a) and (c)) or mean absolute error (MAE) (panel (b)).
Liat Oss-Ronen, Robert A. Redden, and Peter I. Lelkes
Published Online: 26 Aug 2020 https://doi.org/10.1089/scd.2020.0097
Abstract
Directed in vitro differentiation of pluripotent stem cells toward definitive endoderm (DE) offers great research and therapeutic potential since these cells can further differentiate into cells of the respiratory and gastrointestinal tracts, as well as associated organs such as pancreas, liver, and thyroid. We hypothesized that culturing mouse embryonic stem cells (mESCs) under simulated microgravity (SMG) conditions in rotary bioreactors (BRs) will enhance the induction of directed DE differentiation. To test our hypothesis, we cultured the cells for 6 days in two-dimensional monolayer colony cultures or as embryoid bodies (EBs) in either static conditions or, dynamically, in the rotary BRs. We used flow cytometry and quantitative polymerase chain reaction to analyze the expression of marker proteins and genes, respectively, for pluripotency (Oct3/4) and mesendodermal (Brachyury T), endodermal (FoxA2, Sox17, CxCr4), and mesodermal (Vimentin, Meox1) lineages. Culture in the form of EBs in maintenance media in the presence of leukemia inhibitory factor, in static or SMG conditions, induced expression of some of the differentiation markers, suggesting heterogeneity of the cells. This is in line with previous studies showing that differentiation is initiated as cells are aggregated into EBs even without supplementing differentiation factors to the media. Culturing EBs in static conditions in differentiation media (DM) in the presence of activin A reduced Oct3/4 expression and significantly increased Brachyury T and CxCr4 expression, but downregulated FoxA2 and Sox17. However, culturing in SMG BRs in DM upregulated Brachyury T and all of the DE markers and reduced Oct3/4 expression, indicating the advantage of dynamic cultures in BRs to specifically enhance directed DE differentiation. Given the potential discrepancies between the SMG conditions on earth and actual microgravity conditions, as observed in other studies, future experiments in space flight are required to validate the effects of reduced gravity on mESC differentiation.
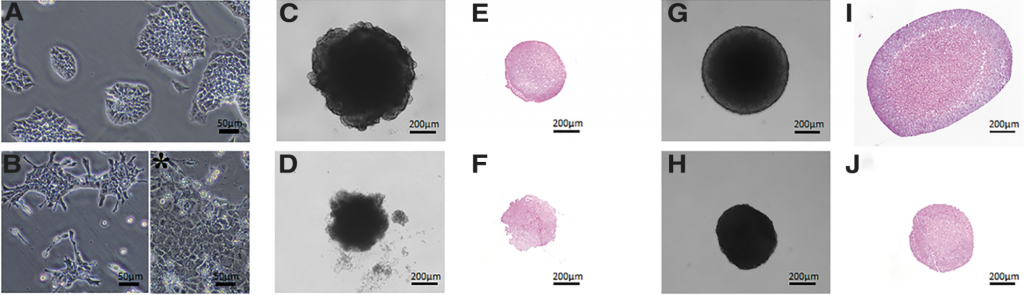
Figure 1: mESC embryoid bodies in culture
See more about our research and a full list of recent publications