Understanding the Structure of Ferrihydrite
Ferrihydrite is a nanoscale iron oxyhydroxide (Fe-O-OH) mineral that is ubiquitous in water and soil systems, and plays an important role in geochemical processes and the fate of contaminants. Ferrihydrite is one of the initial corrosion products in the oxidation of iron, and is the precursor to the formation of the more stable mineral phases goethite (α-FeO(OH)) and hematite (α-Fe2O3 ). Formation occurs by the hydrolysis of ferric species (Fe3+) in solution, resulting in a blood red precipitate. Ferrihydrite is the mineral name given to nanoscale iron oxyhydroxides, but multiple variations exist. The number of reflections observed with X-ray diffraction is conventionally used to differentiate between ferrihydrites with 2-line and 6-line being the most common. It is obvious that the primary difference between 2 and 6-line is the degree of crystallinity, but the ferrihydrite structure is still being debated. Recent synchrotron work involving our laboratory and collaborators at SUNY Stony Brook using total scattering and pair distribution function (PDF) analysis suggests that the primary structure is the same for 2 and 6-line ferrihydrite, Fe10O14(OH)2.
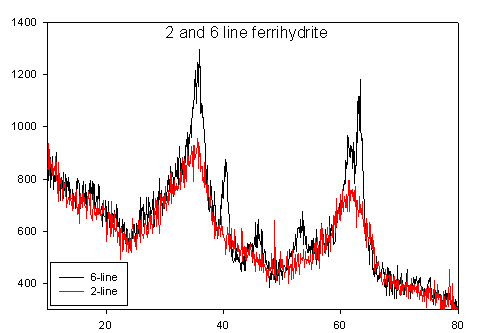
In our laboratory our collaborators and we are continuing the effort to conclusively define the structure of ferrihydrite. To do this we will be using neutron diffraction, which requires us to prepare a synthetic deuterated ferrihydrite. The heavier nucleus of the deuterium will improve scattering and hopefully lead to the decisive determination of structure. The collected diffraction will be analyzed using total scattering and pair distribution function (PDF) analysis, which is sensitive to both short and intermediate range ordering.
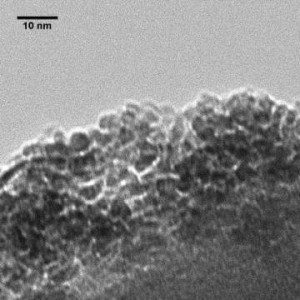
In addition to structure determination we are studying the phase transitions of ferrihydrite, with the goal of determining the transformation mechanisms to hematite and goethite. Goethite is know to grow through the oriented aggregation of ferrihydrite, but the mechanism is yet to be fully understood.
Selected Publications:
Coupled Redox Transformation of Chromate and Arsenite on Ferrihydrite
Cerkez EC.; Bhandari N.; Reeder RJ.; Strongin DR Environmental Science & Technology 49 (5): 2858–2866 (2015)
Photoinduced Oxidation of Arsenite to Arsenate on Ferrihydrite
Bhandari N.; Reeder RJ.; Strongin DR Environmental Science & Technology 45 (7): 2783–2789 (2011)
The Structure of Ferrihydrite, a Nanocrystalline Material
Michel FM.; Ehm L.; Antao SM.; Lee PL.; Chupas PJ.; Liu G.; Strongin DR.; Schoonen MAA.; Phillips BL.; Parise JB. Science 316 (5832): 1726-1729 (2007)
Similarities in 2- and 6-line ferrihydrite Based on Pair Distribution Function Analysis of X-ray Total Scattering Michel FM.; Ehm L.; Liu G.; Han WQ.; Antao SM.; Chupas PJ.; Lee PL.; Knorr K.; Eulert H.; Kim J.; Grey CP.; Celestian AJ.; Gillow J.; Schoonen MAA.; Strongin DR.; Parise JB. Chemistry of Materials 19 (6): 1489-1496 (2007).